 Ion Timing Fidelity under RF exposure: from S4 voltage sensing to mitochondrial ROS and immune dysregulation
Ion Timing Fidelity under RF exposure: from S4 voltage sensing to mitochondrial ROS and immune dysregulation
Cells communicate with electricity as well as chemistry. Voltage‑gated ion channels open and close when a short, positively charged protein segment (called S4) moves inside the membrane’s electric field. Because that sensing region is only about one nanometer across, millivolt‑scale changes are enough to advance or delay channel opening. Under everyday pulsed radiofrequency exposure, those small shifts do not heat tissue, but they do change the timing of potassium, calcium, and proton flux. That timing is the control variable the immune system decodes to decide activation, tolerance, and oxidative killing. We call this control variable ion timing fidelity. When timing fidelity is degraded, mitochondria are pushed toward reactive oxygen species production, innate danger sensors switch on, and tissues drift toward chronic inflammation and reduced tolerance. In organs with many voltage‑gated channels and many mitochondria—heart and nerve—the effects are amplified and match the lesion pattern seen in large animal studies. Indoors we can fix the problem at the signal source: reduce patterning that degrades timing, and move high‑capacity traffic to light‑based networking (LiFi).
The mechanistic chain, end‑to‑end
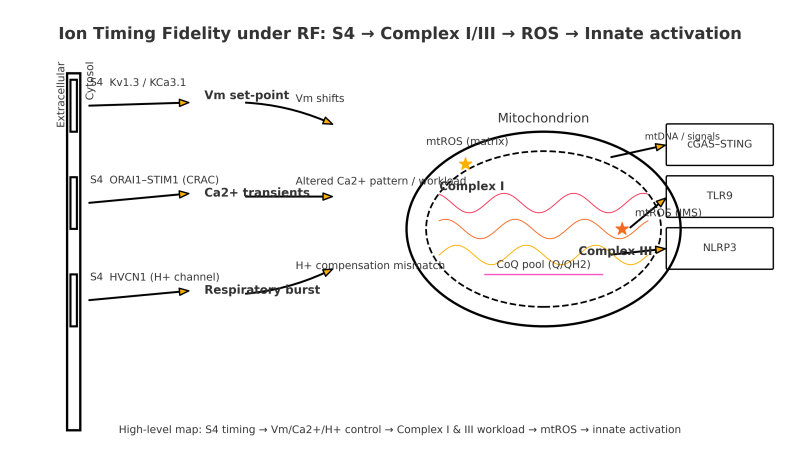
Upstream lever: S4 voltage sensing. The S4 helix carries several positive charges. A change of only tens of millivolts across the roughly one‑nanometer sensing region deterministically shifts the activation energy for opening, advancing or delaying channel gating by large factors on biological time scales. This immediately affects three immune control points:
-
Kv1.3 and KCa3.1 set the membrane potential that maintains the driving force for calcium entry after receptor engagement.
-
ORAI1 with STIM1 (the CRAC complex) generates calcium transients that control NFAT and NF‑kappaB transcription.
-
HVCN1 exports protons to keep the phagocyte respiratory burst within an operative range.
Mitochondrial coupling. Altered calcium patterns and membrane potential change workload at Complex I and Complex III of the electron‑transport chain, the two canonical sites of mitochondrial reactive oxygen species. With sustained pressure, mitochondria increase superoxide and hydrogen peroxide and are more likely to release mitochondrial DNA into the cytosol through permeability pathways. Cytosolic and oxidized mitochondrial DNA are potent ligands for cGAS–STING and TLR9, while redox conditions facilitate NLRP3 assembly. These innate programs then feed back to ion‑channel expression and chemistry, making future S4 transitions even more likely to be mistimed under the same exposure. The system stabilizes into chronic inflammation and low tolerance.
Sufficiency. Mistimed ions and altered membrane potential are sufficient to push immune cells into pro‑inflammatory states. Mitochondrial DNA release is a powerful amplifier and a marker of chronicity, not a prerequisite for ignition.
Why heart and nerve are predicted targets
Neurons and cardiomyocytes pack very high densities of voltage‑gated channels (many S4 sensors per cell) and devote a large fraction of volume to mitochondria. That pairing gives them high electrical sensitivity and high metabolic gain. It is therefore notable that large bioassays reported malignant cardiac schwannomas and brain gliomas under radiofrequency exposures not designed to heat tissue; this tissue pattern is consistent with the ion timing fidelity model.
Evidence convergence along the chain
-
Upstream biophysics is settled. S4 voltage sensing and voltage‑dependent gating are established structural and functional facts (Cell 2019; Neuron 2010).
Links: https://www.cell.com/cell/fulltext/S0092-8674(19)30734-2 ; https://pubmed.ncbi.nlm.nih.gov/20869590/ -
Mid‑chain immune phenotypes appear at realistic exposures. Human monocytic cells exposed to a smartphone‑class signal showed time‑dependent increases in interleukin‑1 alpha, nitric oxide, and superoxide by thirty minutes, reduced phagocytosis at sixty minutes, and partial recovery by one hundred twenty minutes, without loss of viability.
Link: https://pubmed.ncbi.nlm.nih.gov/37207815/ -
Mitochondrial axis is consistently implicated. Reviews of roughly one hundred experiments report oxidative stress at low intensities: reactive oxygen species, lipid peroxidation, and altered antioxidant enzymes. A focused 2018 review on mitochondria details Complex I and III as the primary sources and ties calcium handling to workload under non‑thermal conditions.
Links: https://pubmed.ncbi.nlm.nih.gov/26151230/ ; https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8038719/ ; https://pubmed.ncbi.nlm.nih.gov/30533171/ -
Realistic 5G imprint in brain. A March 2025 mouse study using a smartphone‑class 3.5 GHz 5G NR signal at sub‑thermal specific absorption rates found up‑regulation of ten of the thirteen mitochondrial DNA‑encoded oxidative‑phosphorylation genes in cortex, with enrichment for Complex I and III subunits. Behavior was unchanged, but mitochondrial gene regulation shifted in the expected direction.
Link: https://www.mdpi.com/1422-0067/26/6/2459 -
Organ‑level inflammation in vivo. In rats exposed eight hours per day to mobile‑phone fields for twenty days, bladder histology showed severe inflammatory infiltration; mild infiltration persisted after a twenty‑day rest.
Link: https://pubmed.ncbi.nlm.nih.gov/25251956/ -
Tissue‑selective lesions match the prediction. National Toxicology Program and Ramazzini Institute studies reported heart schwannomas and brain gliomas in male rats under different RF paradigms.
Links: https://www.niehs.nih.gov/research/atniehs/dntp/tox-research/projects/cellphone/index.cfm ; https://pubmed.ncbi.nlm.nih.gov/29530389/
Together, these strands support a coherent, testable chain from S4 gating shifts → ion timing changes → mitochondrial reactive oxygen species → innate activation → chronic inflammation.
“Entropic waste” defined so it is measurable
In this context entropic waste means persistent, non‑native temporal patterning of electromagnetic fields that reduces ion timing fidelity. It is quantified by measurable shifts in channel activation midpoints and open‑time distributions, by altered calcium‑transient statistics, and by mismatches between proton conductance and oxidase output. It is not a metaphor for heat; it is a signal‑level distortion of electrical pacing that biology depends on.
What to do now: a Clean Ether Act
-
Engineer for timing, not just heat. Update performance standards to regulate duty cycle, pulse structure, and peak‑to‑average ratio, alongside proximity and placement.
-
Indoors first. Move high‑capacity traffic to LiFi (IEEE 802.11bb) and maintain wired backbones; remove access points from bedrooms, neonatal spaces, and classrooms where feasible.
-
Restore federal responsibility. Repeal Section 704 of the 1996 Telecommunications Act to allow health‑protective local decisions; enforce Public Law 90‑602 (21 U.S.C. 360hh‑ss, including 360ii and 360kk) so that the Secretary of HHS executes the electronic‑product radiation control program and issues performance standards for consumer RF emitters.
-
Targeted guidance now. Prioritize distance and duration practices for pregnancy and children while standards update.
-
Close the last scientific loop quickly. In the same preparations under indoor‑mimetic pulsing, measure: channel activation parameters; calcium timing and NFAT entry; mitochondrial reactive oxygen species and membrane potential; cytosolic mitochondrial DNA with cGAS–STING, TLR9, and NLRP3 readouts; and demonstrate rescue with pathway‑specific inhibitors and timing‑stabilizing channel modulators.
Small millivolt changes at the S4 voltage sensor change when ion channels open and close. In immune and excitable cells, that resets potassium, calcium, and proton flux, altering NFAT and NF‑kappaB programs and mismatching the respiratory burst. Mitochondria respond with increased reactive oxygen species and, with persistence, release mitochondrial DNA that activates cGAS–STING, TLR9, and NLRP3. Organs rich in channels and mitochondria—heart and nerve—show the strongest macro effects, while other organs can show pure inflammatory phenotypes. This chain is strongly supported at each step by peer‑reviewed evidence and is sufficient to exacerbate diseases driven by oxidative stress, inflammation, or autoimmune factors. Because the driver is timing rather than heat, we can act now: clean the indoor spectrum, set performance standards that protect ion timing fidelity, and shift data traffic to light‑based networking while we complete targeted, timing‑centric studies.
Key links
S4 and gating:
Cell 2019 (resting‑state sodium channel): https://www.cell.com/cell/fulltext/S0092-8674(19)30734-2
Neuron 2010 (voltage sensors review): https://pubmed.ncbi.nlm.nih.gov/20869590/
Immune electrical checkpoints:
Kv1.3/KCa3.1 review: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3253536/
HVCN1 and oxidase coupling: https://www.pnas.org/doi/10.1073/pnas.0902761106
Oxidative stress under RF:
Yakymenko 2016: https://pubmed.ncbi.nlm.nih.gov/26151230/
Schuermann 2021: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8038719/
Monocyte time‑course study 2023: https://pubmed.ncbi.nlm.nih.gov/37207815/
Mitochondria‑focused review 2018: https://pubmed.ncbi.nlm.nih.gov/30533171/
5G cortical mitochondrial signature:
IJMS 2025: https://www.mdpi.com/1422-0067/26/6/2459
Organ‑level inflammation:
Bladder inflammation in rats: https://pubmed.ncbi.nlm.nih.gov/25251956/
Animal signal consistent with susceptibility rule:
NTP RFR program: https://www.niehs.nih.gov/research/atniehs/dntp/tox-research/projects/cellphone/index.cfm
Ramazzini 2018: https://pubmed.ncbi.nlm.nih.gov/29530389/