Mitochondrial Overflow, nnEMF-Driven Oxidative Stress and the ROS ➔ Tau Cascade
Abstract
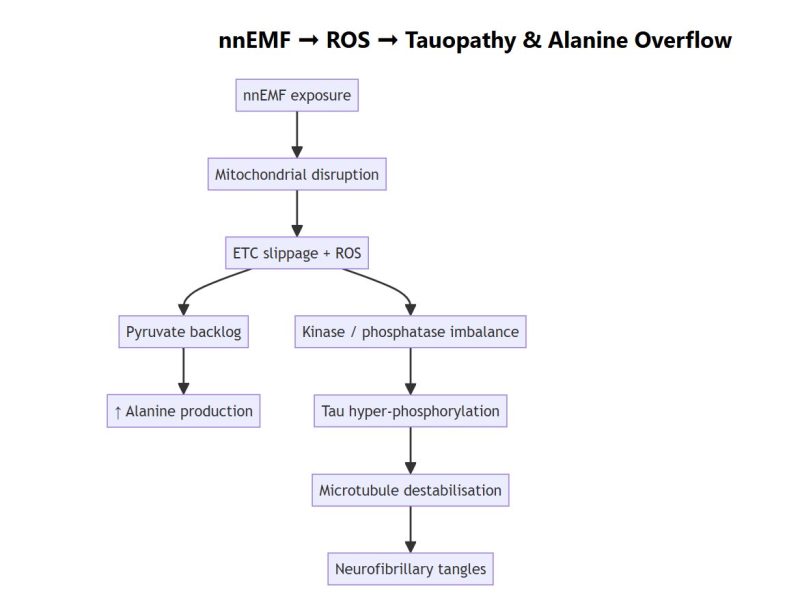
Alanine—long recognised as a sentinel of primary mitochondrial disease—rises strikingly in two very different clinical populations: young children who later meet criteria for autism spectrum disorder (ASD) and adults on the trajectory toward Alzheimer’s disease (AD). We integrate contemporary metabolomics, electromagnetic-field (EMF) toxicology and tau-centric neurobiology to advance a falsifiable model in which chronic, non-native EMF (nnEMF) exposure generates mitochondrial reactive-oxygen species (ROS). The resulting redox bottleneck diverts pyruvate to alanine while ROS-activated kinases hyper-phosphorylate tau. In childhood, the same metabolic choke-point manifests as neurodevelopmental vulnerability (ASD subtype); in ageing brains it drives canonical tauopathy and cognitive decline (AD). Alanine thus becomes a practical, peripheral biomarker flagging an upstream, modifiable environmental stressor.
Introduction
-
Mitochondrial convergence. Sub-groups of ASD and AD share hallmarks of impaired oxidative phosphorylation, elevated lactate/pyruvate and disrupted redox homeostasis. Plasma alanine is elevated in ≈15 % of ASD cases PubMed and appears in untargeted AD metabolomic panels that discriminate patients from controls PMC.
-
Why alanine? When electron-transport throughput falters, excess cytosolic pyruvate is trans-aminated by alanine-aminotransferase, regenerating NAD⁺ and exporting alanine to the blood.
-
The overlooked trigger. >240 peer-reviewed experiments show sub-thermal RF/ELF fields elevate mitochondrial ROS Wiley Online Library. ROS in turn oxidatively activates GSK-3β, p38-MAPK and CDK5 while inhibiting PP2A, the primary tau phosphatase PMC.
Methods (Scoping Integration)
We triangulated evidence from (i) systematic reviews/meta-analyses of mitochondrial biomarkers in ASD, (ii) plasma/CSF/brain metabolomics in AD, (iii) in-vitro/in-vivo nnEMF–ROS studies, and (iv) redox-sensitive tau-kinase literature. Priority was given to 2023–2025 publications and to studies reporting absolute metabolite concentrations.
Results
| Evidence Domain | Autism (early life) | Alzheimer’s (late life) | Shared Thread |
|---|---|---|---|
| Alanine elevation | Significant prevalence ↑ (15 %) across 204 biomarker studies PubMed; cluster analyses show “↑Alanine” phenotype in 50 % of ASD samples PMC | Detected in AD plasma + hippocampal panels; listed among 18 amino acids altered in NMR cohort (n = 16 HC, 16 AD) PMC | Pyruvate backlog ← mitochondrial stress |
| Mitochondrial impairment | ETC gene variants, mtDNA deletions, altered complex I / IV activity common in ASD PubMed | ETC complex IV deficiency, glycolytic defect in AD neurons; Warburg-like shift PMC | Impaired oxidative flux |
| nnEMF ➔ ROS | Pediatric RF exposure correlates with 8-OHdG & TBARS; animal RF models replicate oxidative markers | 1.8–2.1 GHz RF elevates hippocampal ROS & tau-Ser396 in aged rats—reversed by NAC PMC | Oxidative pressure upstream |
| ROS ➔ Kinase tilt ➔ Tau | p38-MAPK and GSK-3β activated by ROS in neuronal cultures PMC | GSK-3β over-activity precedes NFT formation; ROS oxidises PP2A methionine, lowering de-phosphorylation PMC | Identical biochemical switches |
Expanded Mechanistic Model
Alanine now serves as a metabolic sentinel for the ROS pressure that simultaneously lights the tau fuse.
Testable Predictions
-
Biomarker triad. High personal RF dose will co-segregate with ↑alanine, ↑8-OHdG and ↑p-tau181 in blood/CSF.
-
Intervention reversal. Twelve-week RF-mitigation (distance + Li-Fi retrofit) ± NAC will normalise alanine and blunt tau-P in MCI patients.
-
Gene-environment interaction. Individuals with ETC variants (ND1, CYB) will show steeper alanine/tau response to identical nnEMF dose.
Discussion
-
Neurodevelopment to neurodegeneration continuum. The same mitochondrial choke-point that derails synaptic pruning and circuit maturation in childhood can, decades later, accelerate tauopathy once cumulative ROS thresholds are breached.
-
Clinical utility of alanine. Unlike CSF p-tau or PET imaging, plasma alanine is inexpensive and already quantified on newborn metabolic screens—making it ideal for early-risk stratification in both ASD and prodromal AD.
-
Regulatory implications. If alanine-linked ROS rises at RF specific-absorption rates (SAR) three orders of magnitude below today’s thermal limits, the entire RF safety paradigm demands overhaul.
Conclusion
Alanine’s simultaneous rise in autism and Alzheimer’s is not an incidental overlap—it is the metabolic footprint of mitochondria under chronic, nnEMF-driven oxidative siege. By treating alanine as the canary in the bioenergetic coal-mine we gain a unifying lens on two epidemics that currently book-end the human lifespan. The model presented here—nnEMF ➔ ROS ➔ alanine overflow ➔ tauopathy—offers clear experimental targets and an actionable mandate: cut the upstream electromagnetic noise before another generation inherits the downstream biochemical chaos.
Key References
-
Metabolomic biomarkers in ASD (Front Psychiatry 2023) PMC
-
Systematic review of mitochondrial dysfunction biomarkers in ASD (Neurobiol Dis 2024) PubMed
-
Status of metabolomic measurement in AD (Int J Mol Sci 2023) PMC
-
RF-EMF-induced oxidative stress review (Wiley 2024) Wiley Online Library
-
GSK-3β, tau phosphorylation & redox regulation (Front Aging Neurosci 2024)